Feb 9, The ratio of these carbon isotopes reveals the ages of some of Earth’s oldest inhabitants. Cosmic rays bombard Earth’s atmosphere, creating the unstable isotope carbon Radiocarbon dating relies on the carbon isotopes carbon and carbon
Table of contents
- Professor Timothy H. Heaton
- Radiometric dating
- How do geologists use carbon dating to find the age of rocks?
- Age of the Earth - Wikipedia
Boltwood refined his work and finally published the results in Boltwood's paper pointed out that samples taken from comparable layers of strata had similar lead-to-uranium ratios, and that samples from older layers had a higher proportion of lead, except where there was evidence that lead had leached out of the sample. His studies were flawed by the fact that the decay series of thorium was not understood, which led to incorrect results for samples that contained both uranium and thorium.
However, his calculations were far more accurate than any that had been performed to that time. Refinements in the technique would later give ages for Boltwood's 26 samples of million to 2. Although Boltwood published his paper in a prominent geological journal, the geological community had little interest in radioactivity.
Rutherford remained mildly curious about the issue of the age of Earth but did little work on it. Robert Strutt tinkered with Rutherford's helium method until and then ceased.
Professor Timothy H. Heaton
However, Strutt's student Arthur Holmes became interested in radiometric dating and continued to work on it after everyone else had given up. Holmes focused on lead dating, because he regarded the helium method as unpromising.
He performed measurements on rock samples and concluded in that the oldest a sample from Ceylon was about 1. For example, he assumed that the samples had contained only uranium and no lead when they were formed. More important research was published in It showed that elements generally exist in multiple variants with different masses, or " isotopes ". In the s, isotopes would be shown to have nuclei with differing numbers of the neutral particles known as " neutrons ".
In that same year, other research was published establishing the rules for radioactive decay, allowing more precise identification of decay series. Many geologists felt these new discoveries made radiometric dating so complicated as to be worthless. His work was generally ignored until the s, though in Joseph Barrell , a professor of geology at Yale, redrew geological history as it was understood at the time to conform to Holmes's findings in radiometric dating.
Barrell's research determined that the layers of strata had not all been laid down at the same rate, and so current rates of geological change could not be used to provide accurate timelines of the history of Earth. Holmes' persistence finally began to pay off in , when the speakers at the yearly meeting of the British Association for the Advancement of Science came to a rough consensus that Earth was a few billion years old, and that radiometric dating was credible.
Holmes published The Age of the Earth, an Introduction to Geological Ideas in in which he presented a range of 1. No great push to embrace radiometric dating followed, however, and the die-hards in the geological community stubbornly resisted. They had never cared for attempts by physicists to intrude in their domain, and had successfully ignored them so far. Holmes, being one of the few people on Earth who was trained in radiometric dating techniques, was a committee member, and in fact wrote most of the final report.
Thus, Arthur Holmes' report concluded that radioactive dating was the only reliable means of pinning down geological time scales. Questions of bias were deflected by the great and exacting detail of the report. It described the methods used, the care with which measurements were made, and their error bars and limitations. Radiometric dating continues to be the predominant way scientists date geologic timescales. Techniques for radioactive dating have been tested and fine-tuned on an ongoing basis since the s.
Radiometric dating
Forty or so different dating techniques have been utilized to date, working on a wide variety of materials. Dates for the same sample using these different techniques are in very close agreement on the age of the material.
Possible contamination problems do exist, but they have been studied and dealt with by careful investigation, leading to sample preparation procedures being minimized to limit the chance of contamination. An age of 4. The quoted age of Earth is derived, in part, from the Canyon Diablo meteorite for several important reasons and is built upon a modern understanding of cosmochemistry built up over decades of research.
Most geological samples from Earth are unable to give a direct date of the formation of Earth from the solar nebula because Earth has undergone differentiation into the core, mantle, and crust, and this has then undergone a long history of mixing and unmixing of these sample reservoirs by plate tectonics , weathering and hydrothermal circulation.
All of these processes may adversely affect isotopic dating mechanisms because the sample cannot always be assumed to have remained as a closed system, by which it is meant that either the parent or daughter nuclide a species of atom characterised by the number of neutrons and protons an atom contains or an intermediate daughter nuclide may have been partially removed from the sample, which will skew the resulting isotopic date.
To mitigate this effect it is usual to date several minerals in the same sample, to provide an isochron. Alternatively, more than one dating system may be used on a sample to check the date. Some meteorites are furthermore considered to represent the primitive material from which the accreting solar disk was formed.
Nevertheless, ancient Archaean lead ores of galena have been used to date the formation of Earth as these represent the earliest formed lead-only minerals on the planet and record the earliest homogeneous lead-lead isotope systems on the planet. These have returned age dates of 4. Statistics for several meteorites that have undergone isochron dating are as follows: The Canyon Diablo meteorite was used because it is both large and representative of a particularly rare type of meteorite that contains sulfide minerals particularly troilite , FeS , metallic nickel - iron alloys, plus silicate minerals.
This is important because the presence of the three mineral phases allows investigation of isotopic dates using samples that provide a great separation in concentrations between parent and daughter nuclides. This is particularly true of uranium and lead.
How do geologists use carbon dating to find the age of rocks?
Lead is strongly chalcophilic and is found in the sulfide at a much greater concentration than in the silicate, versus uranium. Because of this segregation in the parent and daughter nuclides during the formation of the meteorite, this allowed a much more precise date of the formation of the solar disk and hence the planets than ever before. The age determined from the Canyon Diablo meteorite has been confirmed by hundreds of other age determinations, from both terrestrial samples and other meteorites.
- dating a born again christian woman!
- dream that your dating someone else!
- dhv dating!
- Carbon Dating: The History Of Life On Earth (Video) | HuffPost.
- free dating sites in richmond va!
This is interpreted as the duration of formation of the solar nebula and its collapse into the solar disk to form the Sun and the planets. This 50 million year time span allows for accretion of the planets from the original solar dust and meteorites. The moon, as another extraterrestrial body that has not undergone plate tectonics and that has no atmosphere, provides quite precise age dates from the samples returned from the Apollo missions. Rocks returned from the Moon have been dated at a maximum of 4. Martian meteorites that have landed upon Earth have also been dated to around 4.
Lunar samples, since they have not been disturbed by weathering, plate tectonics or material moved by organisms, can also provide dating by direct electron microscope examination of cosmic ray tracks. The accumulation of dislocations generated by high energy cosmic ray particle impacts provides another confirmation of the isotopic dates. Cosmic ray dating is only useful on material that has not been melted, since melting erases the crystalline structure of the material, and wipes away the tracks left by the particles. Altogether, the concordance of age dates of both the earliest terrestrial lead reservoirs and all other reservoirs within the Solar System found to date are used to support the fact that Earth and the rest of the Solar System formed at around 4.
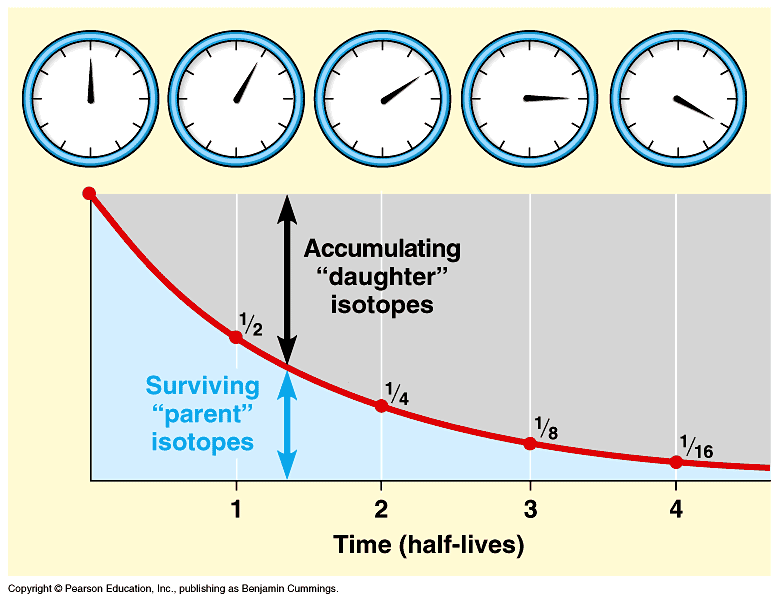
We know that it takes 5, years for half of the C14 in a sample to decay.
- The Age of the Earth - Radiocarbom Dating as a Current Scientific Clock: Jonathan Ring.
- How Carbon-14 Dating Works.
- Navigation menu.
- you have been disconnected from matchmaking hon!
It takes another 5, years for half of what's left to decay, and so on. This is C14's half-life. All radioactive isotopes have one. And if we compare the amount of C14 in a dead thing to the amount of regular carbon, voila! We can find out how old it is. Now, some people who think that the earth is only 6, years old may base their claims on words in the Bible, not measurable evidence. And one ploy they use to cast doubt on radiocarbon dating is to point out its shortcomings. C14 has a relatively short half-life. So, anything older than 50, years only has too little C14 left to make an accurate calculation of its age.
But C14 isn't the only radioisotope out there. There are tons of them!
Age of the Earth - Wikipedia
If I wanted to find out the age of a dinosaur fossil , I might measure its uranium concentration, which has a half-life of million years. Radioactive isotopes like potassium and rubidium have half-lives in the billions of years. Critics also like to point out that over time, the amount of C14 in the Earth's atmosphere may have varied.
But scientists know this , so they make corresponding adjustments to their measurements. And radioisotope dating may be one of the more sophisticated methods we use to know the age of fossils, but it's not the only one. Millions of fossils have been pulled from the earth.
Carbon makes up an extremely small portion of the carbon on earth. In fact, there is about a trillion times more 12 C in the atmosphere than 14 C. When the plant or animal dies, carbon ceases to be absorbed into its tissue. Since 14 C decays over time, and the absorption of all carbon has stopped, the initial condition for a clock is the living ratio of the carbon isotopes. After a certain amount of time, the ratio of 14 C to 12 C, compared to a modern sample of the same type, will give a date for the object in question.
Carbon decays into 14 N through the process called Beta decay with a half-life of approximately 5, years. The beta decay process consists of the atom of 14 C ejecting an electron, or beta particle, out of the nucleus, converting a neutron to a proton in the process. The resulting atom, or daughter product, is 14 N which has the same atomic number, but contains one more proton than the parent product.
A half-life works the same way in any type of decay. In the case of 14 C, every 5, years half of the original 14 C decays into nitrogen. Eventually, there is too little 14 C left in a sample to accurately measure without contamination. Theoretically, radiocarbon techniques have the ability to date samples to around 75, years, but the working threshold of reliable dating is around 50, years.
Samples significantly older than this have very little or even no measurable 14 C left. In order to function properly, natural clocks need an irreversible process that occurs at a constant and known rate. Nuclear decay has a constant rate of decay, but as it turns out, the formation of 14 C in the atmosphere is not always constant.
However, cross-checking techniques such as tree ring dating and coral analysis, 14 C has been reliably calibrated to tens of thousands of years. The newest limit using cross-checking methods is around 26, years Dotinga Carbon isotopes are generally measured through the use of a machine called the accelerated mass spectrometer.
A small portion of the sample is put into the machine which then vaporizes it. Taking advantage of the distinct mass of individual isotopes, the machine distinguishes the 14 C from all of the other atoms and molecules present and is able to count the individual atoms. Charcoal, cloth, bone, or any other material that contains organic carbon can be dated using an accelerated mass spectrometer.